An ingenious method for the determination of the relative and absolute configurations of compounds containing aryl-glycerol fragments by 1H NMR spectroscopy†
Abstract
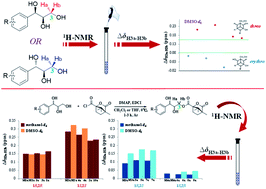
A concise method was established to determine the relative and absolute configurations of aryl-glycerols that depend on the chemical shift differences (Δδ) of the diastereotopic methylene protons (H-3) by 1H NMR spectroscopy. When using DMSO-d6 as the preferred solvent, the threo configuration corresponded to a larger ΔδH3a–H3b value (>0.15 ppm), whereas the erythro configuration (<0.07 ppm) corresponded to a smaller value. Furthermore, the absolute configurations were determined with the aid of a simple acylation reaction through camphanoyl chloride. In the threo enantiomers, the Δδ value of the 1R,2R configuration was <0.15 ppm, and that of the 1S,2S configuration was >0.20 ppm. In the erythro enantiomers, the Δδ value of 1R,2S was >0.09 ppm, and that of 1S,2R was <0.05 ppm. Remarkably, this empirical rule is invalid in CDCl3. In addition, this method was also verified by a quantum 1H NMR calculation.


 Please wait while we load your content...
Please wait while we load your content...