N-doped mixed Co, Ni-oxides with petal structure as effective catalysts for hydrogen and oxygen evolution by water splitting†
Abstract
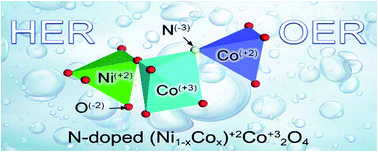
Developing electrocatalytic nanomaterials for green H2 energy is inseparable from the exploration of novel materials and internal mechanisms for catalytic enhancement. In this work, nano-petal N-doped bi-metal (Ni, Co) and bi-valence (+2, +3) (Ni1−xCox)2+Co23+O4 compounds have been in situ grown on the surface of Ni foam. The N3− atoms originate from the amino group in urea and doped in the compound during annealing. The as-synthesized N-doped (Ni1−xCox)2+Co23+O4 nano-petals demonstrate commendable hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) bi-functional catalytic efficiency and stability. Electrochemical measurements confirm that the nitrogen doping significantly improves the catalytic kinetics and the surface area. Density functional theory calculations reveal that the improved HER and OER kinetics is not only due to the synergistic effect of bi-metal and bi-valence, as well as the introduction of defects such as oxygen vacancies, but also it more depends on the shortened bond length between the nitrogen N3− atoms and the metal atoms, and the increased electron density of the metal atoms attached to the N3− atoms. In other words, the change of lattice parameters caused by nitrogen doping is more conducive to the catalytic enhancement than the synergistic effect brought by bi-metal. This study provides an experimental and theoretical reference for the design of bi-functional electrocatalytic nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...