The F19W mutation reduces the binding affinity of the transmembrane Aβ11–40 trimer to the membrane bilayer†
Abstract
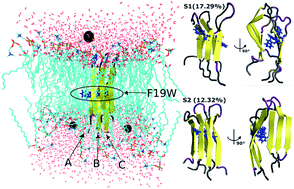
Alzheimer's disease is linked to the aggregation of the amyloid-β protein (Aβ) of 40 or 42 amino acids. Lipid membranes are known to modulate the rate and mechanisms of the Aβ aggregation. Point mutations in Aβ can alter these rates and mechanisms. In particular, experiments show that F19 mutations influence the aggregation rate, but maintain the fibril structures. Here, we used molecular dynamics simulations to examine the effect of the F19W mutation in the 3Aβ11–40 trimer immersed in DPPC lipid bilayers submerged in aqueous solution. Substituting Phe by its closest (non-polar) aromatic amino acid Trp has a dramatic reduction in binding affinity to the phospholipid membrane (measured with respect to the solvated protein) compared to the wild type: the binding free energy of the protein–DPPC lipid bilayer increases by 40–50 kcal mol−1 over the wild-type. This is accompanied by conformational changes and loss of salt bridges, as well as a more complex free energy surface, all indicative of a more flexible and less stable mutated trimer. These results suggest that the impact of mutations can be assessed, at least partially, by evaluating the interaction of the mutated peptides with the lipid membranes.


 Please wait while we load your content...
Please wait while we load your content...