UIO-66-NH2-derived mesoporous carbon used as a high-performance anode for the potassium-ion battery†
Abstract
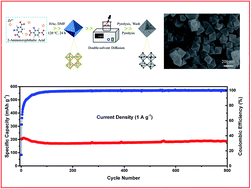
As potassium is abundant and has an electronic potential similar to lithium's, potassium-ion batteries (KIBs) are considered as prospective alternatives to lithium-ion batteries (LIBs). However, the much larger radius of the K ion poses challenges for the potassiation and depotassiation processes when the typical graphite-based anode is used, resulting in poor electrochemical performance. Thus, there is an urgent need to develop novel anode materials that are suitable for K ions. Herein, we develop a porous carbon material with high surface area derived from UIO-66-NH2 metal–organic frameworks as an anode material instead of a graphite-based anode. The material is prepared using a double-solvent diffusion-pyrolysis method, which increased mesopore volume and average pore size, and to a certain extent, slightly improved the nitrogen content of the production. The material exhibits a high capacity as well as excellent rate performance and cycling stability. A potassium battery with our porous carbon as the anode delivers a high reversible capacity of 346 mA h g−1 at 100 mA g−1 (compared to 279 mA h g−1 with a graphite-based anode), and 214 mA h g−1 at a discharge rate of up to 2 A g−1. After 800 cycles, the capacity is still 187 mA h g−1 at 0.1 A g−1. Qualitative and quantitative kinetics analyses demonstrated that the battery's high K storage performance was principally dominated by a surface-driven capacitive mechanism, and the potassiation and depotassiation processes may have occurred on the surface of the porous carbon instead of in the interlayer space, as is the case with a graphite anode. This work may provide a basis for developing other carbonaceous materials to use in KIBs.


 Please wait while we load your content...
Please wait while we load your content...