The design, synthesis, and in vitro trypanocidal and leishmanicidal activities of 1,3-thiazole and 4-thiazolidinone ester derivatives
Abstract
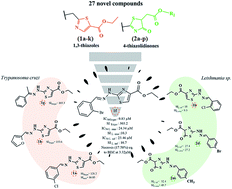
Chagas and leishmaniasis are both neglected tropical diseases, whose inefficient therapies have made them remain the cause for millions of deaths worldwide. Given this, we synthesized 27 novel 1,3-thiazoles and 4-thiazolidinones using bioisosteric and esterification strategies to develop improved and safer drug candidates. After an easy, rapid and low-cost synthesis with satisfactory yields, compounds were structurally characterized. Then, in vitro assays were performed, against Leishmania infantum and Leishmania amazonensis promastigotes, Trypanosoma cruzi trypomastigotes and amastigotes, for selected compounds to determine IC50 and SI, with cytotoxicity on LLC-MK2 cell lines. Overall, 1,3-thiazoles exhibited better trypanocidal activity than 4-thiazolidinones. The compound 1f, an ortho-bromobenzylidene-substituted 1,3-thiazole (IC50 = 0.83 μM), is the most potent of them all. In addition, compounds had negligible cytotoxicity in mammalian cells (CC50 values > 50 μM). Also noteworthy is the examination of the cell death mechanism of T. cruzi, which showed that compound 1f induced necrosis and apoptosis in the parasite. Scanning electron microscopy analysis demonstrated that the treatment of Trypanosoma cruzi trypomastigote cells with the compound 1f at different IC50 concentrations promoted alterations in the shape, flagella and body surface, inducing parasite death. Together, our data revealed a novel series of 1,3-thiazole structure-based compounds with promising activity against Trypanosoma cruzi and Leishmania spp., broadening ways for scaffold optimization.
- This article is part of the themed collection: A celebration of Latin American research in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...