A photoredox/nickel dual-catalytic strategy for benzylic C–H alkoxylation†
Abstract
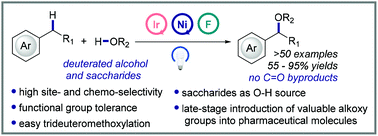
Benzylic C–H alkoxylation via C–H/O–H cross-coupling represents an attractive strategy to access highly functionalized benzylic ethers but remains challenging due to the difficulty in improving the efficiency and selectivity. Herein we wish to report a highly efficient photoredox/nickel dual-catalytic strategy for the site-selective and chemoselective benzylic C–H alkoxylation using readily available Selectfluor as the oxidant. The protocol features broad substrate scope and excellent functional group compatibility and allows the transformation of various of alcohols to pharmaceutically relevant benzylic ethers in moderate to excellent yields under mild reaction conditions. CD3OD was well tolerated, providing a useful synthetic tool for the synthesis of valuable deuterated pharmaceutical molecules. This method is also highlighted by the late-stage introduction of valuable alkoxy groups into pharmaceutical molecules and the structure modification of saccharides.


 Please wait while we load your content...
Please wait while we load your content...