Metal-free electrochemical synthesis of α-ketoamides via decarboxylative coupling of α-keto acids with isocyanides and water†
Abstract
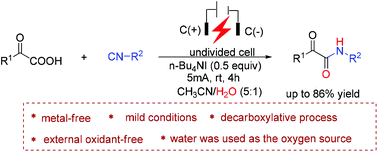
A mild and environmentally benign electrochemical strategy has been developed for the synthesis of α-ketoamides via a decarboxylative coupling reaction of α-keto acids with isocyanides and water. The present reaction was efficiently conducted under constant current electrolysis using n-Bu4NI as the electrolyte in an undivided cell. A series of α-ketoamides could be conveniently and rapidly constructed at room temperature in the absence of transition metal catalysts and external oxidants. Mechanistic studies suggested that water not only served as a starting material to construct α-ketoamides, but also played an important role in promoting the electrochemical redox of α-keto acids.


 Please wait while we load your content...
Please wait while we load your content...