Synthesis of N-indolated amino acids or peptides from 2-alkynylanilines via a dearomatization process†
Abstract
A one-pot stepwise procedure for the synthesis of N-indolated amino acids or peptides from readily available 2-alkynylanilines was reported. The protocol involves the oxidative dearomatization of 2-alkynylanilines, the imino exchange with aliphatic amines, and subsequent cascade cyclization/nucleophilic addition/aromatization. The synthetic method might allow a rapid access to indolactam alkaloid derivatives.
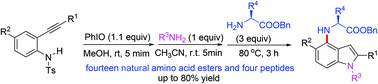


 Please wait while we load your content...
Please wait while we load your content...