An MeSeSO3Na reagent for oxidative aminoselenomethylation of maleimides†
Abstract
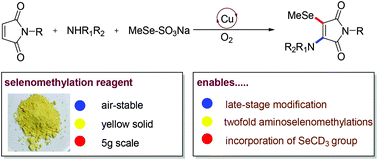
Herein, we describe the design and synthesis of an MeSeSO3Na reagent, which proved to be a versatile selenomethylation reagent for copper-catalyzed aminoselenomethylation of maleimides. This simple and efficient catalytic system is applicable for late-stage vinylselenomethylation of secondary amine-containing pharmaceuticals and two-fold aminoselenomethylation reactions. Most importantly, the current strategy provides a direct and convenient method for the incorporation of the SeCD3 group.


 Please wait while we load your content...
Please wait while we load your content...