Synthesis of δ-phosphorothiolated alcohols by photoredox/copper catalyzed remote C(sp3)–H phosphorothiolation of N-alkoxypyridinium salts†
Abstract
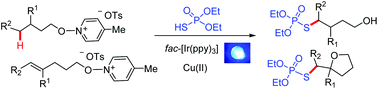
Incorporation of the (RO)2P(O)S group through unreactive C(sp3)–H phosphorothiolation remains a challenging area of research. Under mild photoredox/copper catalysis, the reaction of readily available (EtO)2P(O)SH with N-alkoxypyridinium salts afforded δ-phosphorothiolated alcohols in good yields. The δ-C(sp3)-phosphorothiolation reaction went through a cascade process involving photo-promoted alkoxy radical generation, 1,5-hydrogen atom transfer, and Cu-catalyzed cross-coupling of the δ-C(sp3)-centered radical with (EtO)2P(O)SH. In addition, this photoredox/copper system also enabled O-radical ring-closure and phosphorothiolation from N-alkenyloxypyridinium salts to form β-phosphorothio tethered tetrahydrofurans.


 Please wait while we load your content...
Please wait while we load your content...