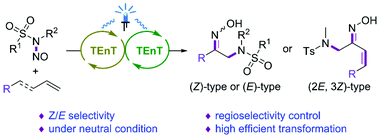
Controllable Z/E-selective synthesis of α-amino-ketoximes from N-nitrososulfonamides and aryl alkenes under neutral conditions†
Abstract
Reported herein is the design of a photosensitization strategy to generate the unique triplet state reactivity of N-nitrososulfoximides, specifically N-methyl-N-nitroso-p-toluenesulfonamide (NANS1), and the subsequent installation of both N-centered sulfonamidyl radical and nitric oxide functionalities into aryl alkene feedstocks, which are mild and completely atom economical, exhibit broad functional group tolerance, and occur readily under neutral conditions. Furthermore, this newly developed methodology is also compatible with both terminal and internal 1,3-dienes to afford solely 1,2-amidoximation products in a remarkable regio- and Z/E-selective manner. Compared with traditional methods that are based on the steric hindrance of the substrates to control the reaction selectivity, both the (E)- and (Z)-isomers of α-amino-ketoximes can be obtained selectively by the switch of sensitizers in our protocol. The key photophysical properties of NANS1 and DFT calculations were investigated in detail, providing the fact that the reaction proceeds through a sensitized triplet energy transfer pathway.
- This article is part of the themed collection: 2021 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...