Amino-assisted synthesis of alkynylthioethers via a visible-light-induced C(sp)–SII coupling between bromoalkynes and 2,2′-diaminodiaryldisulfides†
Abstract
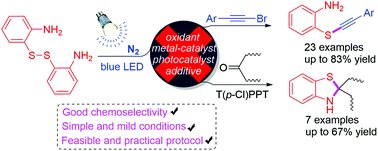
A straightforward synthesis of alkynylthioethers via an amino-assisted and visible-light-promoted coupling reaction of bromoalkynes with 2,2′-diaminodiaryldisulfides has been developed. The reaction can be accomplished in the absence of transition metals, photocatalysts and additives, providing a wide variety of alkynylthioethers in good yields. While switching bromoalkynes to aliphatic ketones, a visible-light-induced cascade cyclization is established to afford dihydrobenzothiazoles using pyrylium salt as an effective photocatalyst.


 Please wait while we load your content...
Please wait while we load your content...