Chemoselective acylation of N-acylglutarimides with N-acylpyrroles and aryl esters under transition-metal-free conditions†
Abstract
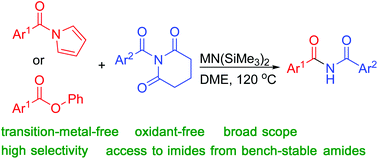
The imide moiety is a well-known structural motif in bioactive compounds and a useful building block in a variety of processes. Using N-acylglutarimides with MN(SiMe3)2 and either N-acylpyrroles or aryl esters, an operationally convenient method to produce a wide array of diaryl- and alkyl arylimides is presented. Symmetric imides are also accessible when N-acylglutarimides are employed as acylation reagents under similar reaction conditions. A unique feature of this method stems from the use of two different electrophilic acylating reagents leading to the formation of the unsymmetrical imides with excellent chemoselectivity.


 Please wait while we load your content...
Please wait while we load your content...