Site-selective binding of terpenoids within a confined space of metal–macrocycle framework: substrate-specific promotion or inhibition of cyclization reactions†
Abstract
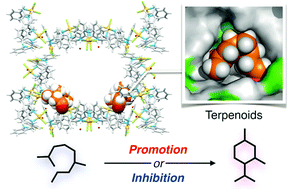
Site-selective adsorption of four terpenoids, (S)-citronellal, nerol, geraniol and farnesol, in a porous metal–macrocycle framework (MMF) was revealed by single-crystal X-ray diffraction analyses. When the cyclic reactions of these terpenoids were investigated, the cyclization reaction of (S)-citronellal was significantly promoted by MMF, but other cyclization reactions were completely suppressed even under the condition that an acid catalyst was fixed on the channel surface of MMF. Furthermore, in the reaction of the terpenoid mixture, (S)-citronellal was substrate-specifically cyclized. The promotion or inhibition of the terpenoid cyclization could be explained based on the difference in their binding structures to these MMF channels.
- This article is part of the themed collection: Macrocycle-based Supramolecular Elements


 Please wait while we load your content...
Please wait while we load your content...