Organocatalytic domino annulation of in situ generated tert-butyl 2-hydroxybenzylidenecarbamates with 2-isothiocyanato-1-indanones for synthesis of bridged and fused ring heterocycles†
Abstract
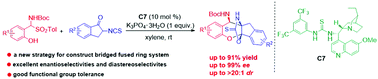
A one-pot efficient asymmetric domino annulation of 2-isothiocyanato-1-indanones with tert-butyl 2-hydroxybenzylidenecarbamates in situ generated from 2-hydroxyaryl-substituted α-amido sulfones was developed. This reaction firstly provided a powerful tool for the enantioselective construction of functionalized bridged fused ring hererocycles bearing three adjacent stereogenic centers in high yields with excellent diastereo- and enantioselectivities (up to 91% yield, >20 : 1 dr and 99% ee).


 Please wait while we load your content...
Please wait while we load your content...