Construction of novel bridged aromatic ring-fused oxazocine frameworks via an N-heterocyclic carbene-catalyzed azabenzoin reaction and radical-initiated cascade cyclization†
Abstract
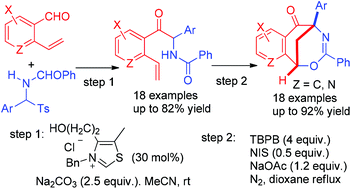
A novel and efficient method for the construction of 1,5-methanobenzo[f][1,3]oxazocin-6-one compounds from o-vinyl benzaldehydes and N-acylarylimines has been developed. The synthesis proceeded through the sequential NHC-catalyzed azabenzoin reaction and radical-initiated regioselective intramolecular cascade cyclizations. This protocol features mild conditions, good functional group tolerance and high yields of products. Novel 6,10-methanopyrido[3,2-f][1,3]oxazocin-5-ones could also be synthesized from 2-vinylnicotinaldehyde and N-acylarylimines based on this method. Capitalizing on the operational simplicity and use of efficient C–C and C–X bond-forming reactions, this protocol combining NHC-catalyzed and radical-initiated reactions enables the assembly of bridged aromatic ring-fused oxazocine derivatives with versatile functional and structural diversities.
- This article is part of the themed collection: FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...