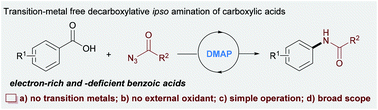
Transition-metal-free decarboxylative ipso amination of aryl carboxylic acids†
Abstract
A convenient DMAP-catalysed decarboxylative amination of aryl and alkyl carboxylic acids is developed under transition-metal-free conditions. This reaction allows the sustainable synthesis of a wide range of aryl and alkyl amines in useful to good yields, avoiding the use of any activating reagent, transition-metal catalyst and external oxidant. The late-stage modification of bioactive acids and concise synthesis of carbamate and ureas demonstrated the synthetic potential of this methodology.


 Please wait while we load your content...
Please wait while we load your content...