Palladium-catalyzed C8–H arylation and annulation of 1-naphthalene carboxylic acid derivatives with aryl iodides†
Abstract
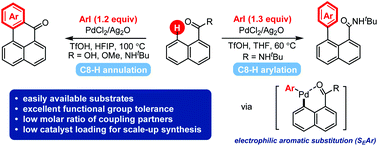
The discovery of a concise route enabling site-selective arylation of polycyclic aromatic hydrocarbons (PAHs) with easily available aryl sources is an appealing yet challenging task toward the bottom-up preparation of π-extended PAHs. Disclosed herein is a palladium-catalyzed C8–H arylation of 1-naphthoic acid derivatives with aryl iodides in a low reactant molar ratio via an electrophilic aromatic substitution (SEAr) process. In addition, a C8–H annulation protocol is developed to rapidly assemble benzanthrones by simply switching the solvent to 1,1,1,3,3,3-hexafluoropropan-2-ol (HFIP).


 Please wait while we load your content...
Please wait while we load your content...