Organocatalytic stereoselective 1,6-addition of thiolacetic acids to alkynyl indole imine methides: access to axially chiral sulfur-containing tetrasubstituted allenes†
Abstract
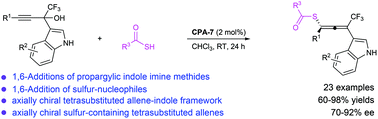
A chiral phosphoric acid-catalyzed enantioselective 1,6-conjugate addition of thiolacetic acid to alkynyl indole imine methide in situ formed from α-(3-indolyl) propargylic alcohol has been established, which enables the formation of axially chiral sulfur-containing tetrasubstituted allenes in generally high efficiency with stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...