Abstract
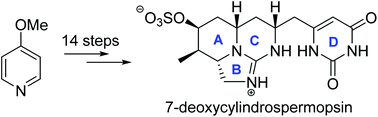
7-Deoxycylindrospermopsin is a member of cylindrospermopsin marine alkaloid containing a cyclic guanidine group. Herein, we present a concise formal total synthesis of (±)-7-deoxycylindrospermopsin in 14 steps from commercailly available 4-methoxypyridine. Key features of our synthetic route include the elaboration of densely functionalized piperidine via 1,4-addition of propargylindium to enone, Sonogashira coupling to attach the pyrimidine moiety, and intramolecular conjugate addition of guanidine with alkyne to form C ring. The synthesis of its 8-epi isomer was also achieved following the same synthetic strategy.


 Please wait while we load your content...
Please wait while we load your content...