Radical 1,2-addition of bromoarenes to alkynes via dual photoredox and nickel catalysis†
Abstract
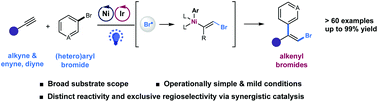
A regioselective, intermolecular 1,2-addition of aryl bromides to alkynes enabled by the photocatalytic generation of bromine radicals via photoredox and nickel catalysis is reported. This mild and redox-neutral protocol tolerates a wide range of (hetero)aryl bromides as well as alkynes, diynes, and enynes, generating diverse alkenyl bromides with exclusive regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...