Copper-catalyzed thioamination of maleimides with diethylphosphorodithioate and amines†
Abstract
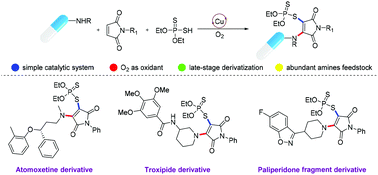
The first copper-catalyzed oxidative aminophosphorothiolation of maleimides with secondary alkylamines and diethylphosphorodithioate has been established. This reaction, featuring a simple, efficient catalytic system and excellent functional group tolerance, provides a concise pathway to access structurally diverse β-amino vinyl phosphorodithioates. The utility of the current protocol is further highlighted in late-stage vinylphosphorothiolation of amine-containing drug fragments.


 Please wait while we load your content...
Please wait while we load your content...