Synergetic copper/TEMPO-catalysed benzylic C–H imidation with N-fluorobenzenesulfonimide at room temperature and tandem conversions with alcohols or arenes†
Abstract
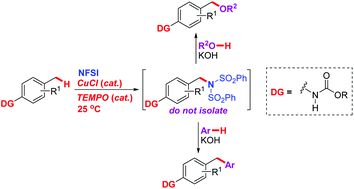
A remote carbamate-directed benzylic C–H imidation at room temperature with N-fluorobenzenesulfonimide (NFSI) through synergetic CuCl and nitroxyl radical 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) catalysed radical relay, as well as tandem benzylic imidation–alkoxylation and imidation–arylation via C(sp3)–N cleavage without isolation of the imidation products, is achieved, providing a facile and efficient strategy for diversified construction of C(sp3)–N, C(sp3)–O and C(sp3)–C(sp2) bonds through C(sp3)–H cleavage under mild conditions. Probable mechanisms are proposed according to the results of control experiments, and the carbamate directing group is proved to be crucial to both the imidation and the alkoxylation/arylation processes. Applications of these tandem reactions in the preparation of key intermediates for the synthesis of bioactive molecules are also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...