The Piancatelli rearrangement of non-symmetrical furan-2,5-dicarbinols for the synthesis of highly functionalized cyclopentenones†
Abstract
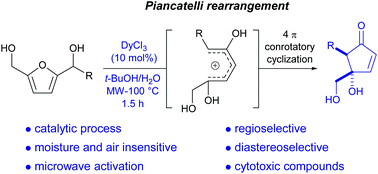
Substituted and non-symmetrical furan-2,5-dicarbinols were readily prepared from hydroxymethylfurfural (HMF), a renewable raw material from biomass. The Piancatelli rearrangement of these furan-2,5-dicarbinols, catalyzed by DyCl3 and under microwave activation, affords 5-substituted-4-hydroxymethyl-4-hydroxycyclopentenones in a regio- and diastereoselective manner. This methodology can be extended to aza-Piancatelli type reactions by using nitrogen nucleophiles, to furnish the corresponding aminocyclopentenone derivatives. These synthetized bis-hydroxylated cyclopentenone derivatives exhibited significant cytotoxicity against eight human tumor cell lines.


 Please wait while we load your content...
Please wait while we load your content...