Decarboxylative C–H alkylation of heteroarenes by copper catalysis†
Abstract
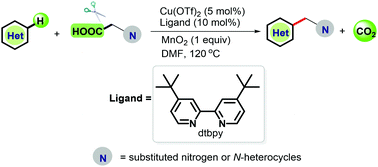
Versatile decarboxylative C–H alkylation of heteroarenes was accomplished. In the presence of Cu(OTf)2 and 4,4′-di-tert-butyl-2,2′-bipyridine, a range of heteroarenes, such as imidazo[1,2-a]pyridines, 2-phenylbenzo[d]imidazo[2,1-b]thiazole, 2-phenylindolizine and 4H-chromen-4-one, can be alkylated using diverse alkyl carboxylic acids. This developed protocol will extend the still limited number of copper catalytic decarboxylation couplings, especially in the construction of Csp2–Csp3 bonds.


 Please wait while we load your content...
Please wait while we load your content...