Pd(ii)-Catalyzed enantioconvergent twofold C–H annulation to access atropisomeric aldehydes: a platform for diversity-oriented-synthesis†
Abstract
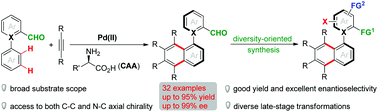
Herein, we present the first Pd(II)-catalyzed atroposelective dual C–H annulative strategy for the assembly of axially chiral aldehyde frameworks using commercially available amino acid as the transient auxiliary and chiral pool. This reaction accommodates a broad substrate scope to give atropisomeric aldehydes bearing both C–C and N–C chiral axes in good yields (up to 95%) with excellent enantioinduction (up to 99%). The utility of this synthetic methodology was testified by various practical late-stage transformations, thereby accomplishing diversity-oriented synthesis of structurally diverse biaryl atropisomers and several functionalized axially chiral species such as bifunctional organocatalysts. Moreover, a series of mechanistic studies have provided more details for this catalytic transformation.


 Please wait while we load your content...
Please wait while we load your content...