Highly efficient oxidative cleavage of olefins with O2 under catalyst-, initiator- and additive-free conditions†
Abstract
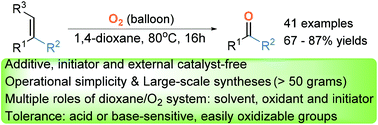
Described are the first examples of 1,4-dioxane-promoted oxidative cleavage of olefins to carbonyls with atmospheric oxygen as the sole oxidant and the maximum conversion was obtained up to 95%. This reaction is external catalyst-, initiator- and additive-free with excellent functional group tolerance (base and oxidant sensitive groups) and is operationally simple. It also provides a practical protocol for exceptional efficiency, large-scale synthesis (>50 grams), late-stage modification of complex poly-functionalized molecules, and one-pot sequential transformations starting from alkenes.


 Please wait while we load your content...
Please wait while we load your content...