Palladium-catalyzed directing group assisted and regioselectivity reversed cyclocarbonylation of arylallenes with 2-iodoanilines†
Abstract
A palladium-catalyzed regioselective cyclocarbonylation of N-(2-pyridyl)sulfonyl (N-SO2Py)-2-iodoanilines with allenes has been developed. With the assistance of the directing group N-SO2Py, the regioselectivity of arylallenes was reversed and the reaction proceeded well to give various 3-methylene-2,3-dihydroquinolin-4(1H)-ones with up to 95% yield by using benzene-1,3,5-triyl triformate (TFBen) as the CO source. Control experiments and DFT calculations were performed to understand the reaction details and a possible reaction mechanism is proposed accordingly.
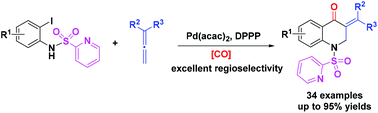


 Please wait while we load your content...
Please wait while we load your content...