Remote methylene C(sp3)–H functionalization enabled by organophosphine-catalyzed alkyne isomerization†
Abstract
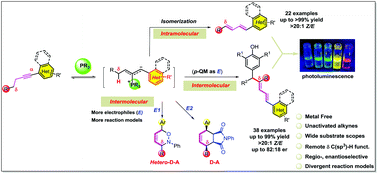
A remote δ methylene C(sp3)–H functionalization catalyzed by an organophosphine through an alkyne isomerization/conjugate addition cascade was discovered. No classical electron-withdrawing group is required to pre-activate the aryl alkyne, which will undergo isomerization to its corresponding diene under only 10 mol% of PMe2Ph with a catalytic proton shuttle. The phosphoryl ylide was postulated as a key intermediate and different electrophiles were used to trap the vinylogous ylide that resulted in a large group of δ C(sp3)–H functionalized products (>60 examples, yields up to 99%) with good diastereoselectivity. Mechanistic studies were carried out and a catalytic cycle was proposed based on deuterium-labeling experiments. The conjugated polyene products were investigated for their fluorescence properties.


 Please wait while we load your content...
Please wait while we load your content...