Arylation of enelactams using TIPSOTf: reaction scope and mechanistic insight†
Abstract
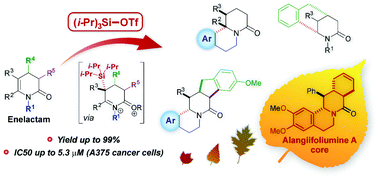
A novel method for the inter- and intramolecular arylation of enelactams (3,4-dihydropyridin-2-ones), up to 99% yield, triggered by triisopropylsilyltrifluoromethanesulfonate (TIPSOTf) is proposed. It offers high synthetic usefulness, especially for the synthesis of polycyclic systems obtained from conformationally rigid δ-enelactams, for which the use of the conventional method, involving cyclization by treatment with TfOH, failed. Multinuclear (1H, 13C, 19F and 29Si) NMR spectroscopy applied for the reaction monitoring as well as DOSY NMR experiment permitted identification of the intermediates and proposition of the reaction mechanism. In the process of checking the scope of the method application, condensed and bridged polycyclic piperidin-2-ones, including a derivative of alangiifoliumine A, were obtained. The inhibition of malignant melanoma A375 cell proliferation by some benzoquinolizidine derivatives (up to IC50 = 5.3 ± 0.4 μM) was evidenced.


 Please wait while we load your content...
Please wait while we load your content...