Carbene-catalyzed selective addition of isothioureas to enals for access to sulphur-containing 5,6-dihyropyrimidin-4-ones†
Abstract
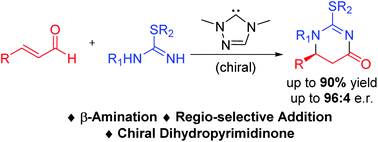
A carbene-catalyzed addition reaction between isothioureas and enals under oxidative conditions is reported. The key steps of our reactions involve the formation of two carbon–nitrogen bonds in a highly regio-selective manner. A variety of sulphur-containing 5,6-dihydropyrimidin-4-ones are obtained with high optical purity.


 Please wait while we load your content...
Please wait while we load your content...