Cp*Ir(iii)- and Cp*Rh(iii)-catalyzed C(sp2)–H amination of arenes using thioethers as directing groups†
Abstract
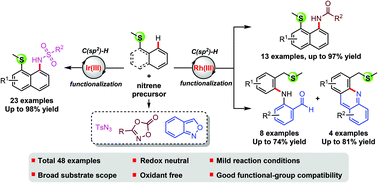
A mild and selective Cp*Ir(III)- and Cp*Rh(III)-catalyzed direct C(sp2)–H amination of arenes and three types of nitrene precursor reagents is reported, with the assistance of a thioether directing group. This redox-neutral protocol proceeds under mild reaction conditions, has a broad substrate scope and high functional-group compatibility and generates the desired aminated products in moderate to excellent yields. The reaction represents the first example of a thioether-directed Cp*Ir(III)- and Cp*Rh(III)-catalyzed C(sp2)–H direct amination reaction. Moreover, azide compounds were employed as a diverse and robust aminating reagent for the first time in a thioether-directed C–H functionalization reaction.


 Please wait while we load your content...
Please wait while we load your content...