Iridium-catalyzed asymmetric hydrogenation of N-phosphinoylimine†
Abstract
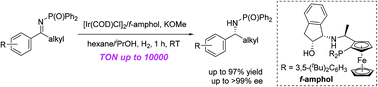
Enantioselective hydrogenation of imines involving an outer-sphere mechanism was achieved. On catalysis with an iridium tridentate catalyst, prochiral N-phosphinoylimines were hydrogenated with high enantioselectivities. The turnover number could reach 10 000. This method could be applied in the synthesis of chiral amines with an α-stereocenter. Indeed, cinacalcet was successfully prepared with a remarkably high enantioselectivity using this method.


 Please wait while we load your content...
Please wait while we load your content...