Alkylaminomaleimide fluorophores: synthesis via air oxidation and emission modulation by twisted intramolecular charge transfer†
Abstract
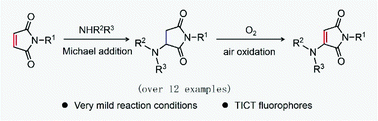
In this work, the synthesis and modulation as well as the mechanisms of a series of 3-aminomaleimide fluorophores based on the air oxidation of 3-aminosuccinimides are reported through combined experimental and computational studies. The central observations include the following: (1) the evident fluorescence observed in the Michael addition reaction of amine to maleimide was attributed to the formation of 3-aminomaleimide fluorophores via the air oxidation process, (2) the chemical conversion from aminosuccinimide to aminomaleimide is thermodynamically favourable and a series of primary and secondary amines are compatible with this reaction, and (3) the steric and electronic effects exerted by amino substituted groups can effectively inhibit twisted intramolecular charge transfer (TICT) and markedly improve the quantum yields of 3-aminomaleimide fluorophores. As a proof-of-concept, the 3-aminomaleimides manifested themselves as efficient fluorophores that can be readily applied in solid emission for optical waveguides and emissive oligomers for bioimaging. Our findings establish a new frontier on the innovative synthesis and accurate modulation of simple luminescent structures for versatile, high-performance functional materials.


 Please wait while we load your content...
Please wait while we load your content...