Enantioselective synthesis of chiral tetrasubstituted allenes: harnessing electrostatic and noncovalent interactions in a bifunctional activation model for N-triflylphosphoramide catalysis†
Abstract
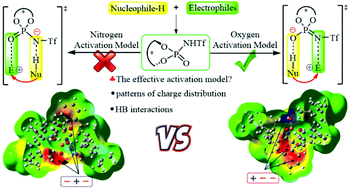
The effect of electrostatic and noncovalent interactions on the activation model and enantioselectivity for N-triflylphosphoramide catalysis was quantified using density functional theory (DFT) calculations on the basis of C–C and C–S bond formation case reactions. Unexpectedly, the computations reveal that the oxygen activated model ([O−⋯H–Nu]) is more effective than the nitrogen activated model ([N−⋯H–Nu]) for N-triflylphosphoramide catalysis. The different chiral electrostatic environment of the chiral pocket controlled by the patterns of charge distribution for the functional group may distinguish the preferred activation model by creating associate noncovalent interactions, which is also responsible for the enantioselectivity in the title reaction. Furthermore, the free hydroxyl substituent can slightly enhance the enantiocontrol through contributing relative favorable distortion energy as compared to the methyl substituted case. Our general applicability finding not only provides a general effective mode for understanding the enantioselectivity under N-triflylphosphoramide catalysis, but should also guide the development of more effective asymmetric organocatalysts.


 Please wait while we load your content...
Please wait while we load your content...