Electrochemically promoted C-3 amination of 2H-indazoles†
Abstract
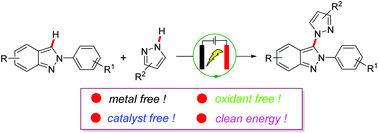
This paper reports a metal-free and external oxidant-free electrochemical method for the C-3 amination of 2H-indazoles. The protocol employs commercially available azoles and aliphatic amines as amination reagents and has a broad substrate scope, providing the corresponding C3-amination products in high yields at room temperature. Mechanistic studies indicate that an oxidative addition/elimination sequence is involved under electrochemical conditions.


 Please wait while we load your content...
Please wait while we load your content...