Dual anionic substitution engineering for an advanced NASICON phosphate cathode in sodium-ion batteries†
Abstract
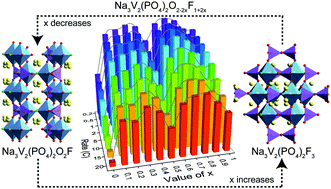
Sodium-ion batteries (SIBs) are prominently used for stationary energy storage due to the abundant resources and low cost of Na. The development of high-performance cathodes for SIBs will be a favorable choice for competing with the market-dominant lithium-ion batteries. Among the various cathodes, Na3V2(PO4)2O2−2xF1+2x (0 ≤ x ≤ 1) materials have become a preferred choice due to their superior structural stability, fast ion transport and high operating potential. Herein, a series of materials with various ratios of F− and O2− (F/O) are prepared via a high temperature solid-state method, and the tuning mechanism of different F/O ratios is studied in detail by analyzing the structural evolution, electrochemical performance and reaction kinetics of materials. The optimal F/O ratio material Na3V2(PO4)2O0.6F2.4 (x = 0.7) exhibits a favorable rate and cycling performance. The capacity at 20C is equivalent to that of the x = 0 material at 5C, and each cycle decay is 0.040% after 200 cycles at 0.5C. Moreover, the optimized F/O ratio material (x = 0.7) also demonstrates excellent reaction kinetics, and the Na apparent diffusion coefficient (Dapp,Na) for the high potential region is about 10−10–10−12 cm2 s−1. A systematic research of dual anion substitution in phosphates will be useful for the structural design and performance improvement of other cathode materials in SIBs.


 Please wait while we load your content...
Please wait while we load your content...