Cyano-containing tetraphenylethene isomers: similar bright mechanoluminescence, but diverse recoverable processes†
Abstract
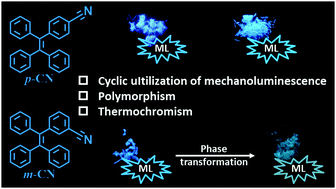
Two simple luminogens p-CN and m-CN were obtained by introducing a cyano group at the para- and meta- position of the tetraphenylethene (TPE) skeleton, respectively. The two luminogens exhibited bright blue mechanoluminescence (ML) even under subdued light both in their crystalline and as-prepared powder state. They realized in situ recovery of ML with a simple thermal treatment, but with diverse recovery processes: p-CN returned to its original blue fluorescence, while m-CN transformed into cyan fluorescence. Molecule p-CN crystallized into a single crystal p-CN-c with blue emission, while m-CN crystallized into two polymorphs, m-CN-c1 and m-CN-c2, with blue emission and cyan emission, respectively. Crystallography analysis suggested that their ML was due to the interlocked molecular packing and intact three-dimensional supramolecular networks stabilized by the multiple strong intermolecular interactions. Their diverse recovery processes upon thermal stimuli were ascribed to their different transition path between their polymorphs. p-CN returned to molecular packing similar to its single crystal, while m-CN irreversibly transformed from packing similar to crystal m-CN-c1 into that of m-CN-c2, which had lower energy and a more twisted conformation. These results provide an important insight for better deciphering the relationship between ML and molecular packing, and help to optimize the rational molecular design of ML materials.


 Please wait while we load your content...
Please wait while we load your content...