Oxygen vacancy enriched Cu-WO3 hierarchical structures for the thermal decomposition of ammonium perchlorate†
Abstract
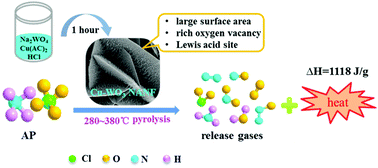
The design and fabrication of efficient catalysts for ammonium perchlorate (AP) decomposition is crucial for the performance of composite solid propellants. Herein, a novel hierarchical structure material of Cu-WO3 nanowire arrays on a nanoflower flake (Cu-WO3 NANF) was synthesized by a facile hydrothermal reaction. The negatively charged (001) polar facets of hexagonal WO3 and the action of Cu2+ induced growth were two important factors for the formation of hierarchical structures. The Cu-WO3 NANF exhibited remarkable catalytic activity for the thermal decomposition of AP. The temperature and activation energy of high temperature AP decomposition were significantly decreased to 378.3 °C and 147.01 kJ mol−1, respectively, which are attributed to the more oxygen vacancies and lower band gap energy of the Cu-WO3 NANF. Thus, it was excited at a lower heat to produce activated species. Under the strong adsorption of Cu-WO3 NANF surface Lewis acid, the activated species can react with NH3 quickly and deeply to produce N2, N2O, NO2 and NO gases, accompanied by a heat release of up to 1118 J g−1. The proposed catalytic mechanism was further corroborated by the in situ TG-MS result. This facile strategy provided a new idea for the design of hierarchical catalysts, and our research opened up a new field for WO3 applications.


 Please wait while we load your content...
Please wait while we load your content...