Does the intrinsic photocontrollable oxidase-mimicking activity of 2-aminoterephthalic acid dominate the activity of metal–organic frameworks?†
Abstract
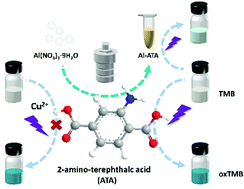
Metal–organic frameworks (MOFs) are a promising type of candidate for mimicking natural enzymes, where ligands play an important role, but are rarely considered. This work proves that the ligand 2-aminoterephthalic acid (ATA) with photoresponsive oxidase-like activity could catalyze the substrate 3,3′,5,5′-tetramethylbenzidine (TMB) to form blue oxTMB under ultraviolet light illumination, and the oxidation reaction could be initiated, suspended, or terminated by light control, ensuring the precision of the spatial and temporal resolution. The presumable mechanism of enzyme-like activity is demonstrated to be relevant to the formation of superoxide radicals (O2˙−) by photogenerated electron transfer. However, once Cu2+ ions are added to the ATA catalytic chromogenic system, the photooxidase-like activity of ATA could be inhibited due to the electron transfer and the formation of Cu-MOF, establishing a photoresponsive colorimetric sensor of Cu2+. In light of the role of ATA as a ligand in the MOF synthesis and the unique characteristics of MOFs, this paper reports an aluminum-based MOF made of ATA (Al-ATA), which has no oxidase-mimicking activity under dark conditions but shows photooxidase-like activity, confirming the importance of light for enzyme mimicking activity and throwing light on the exploration of the construction of ATA-based MOFs (ATA-MOFs) with photocontrollable oxidase mimicking activity as well as the possible photocatalytic mechanism of the MOFs.


 Please wait while we load your content...
Please wait while we load your content...