Nonclassical oxygen atom transfer reactions of an eight-coordinate dioxomolybdenum(vi) complex†
Abstract
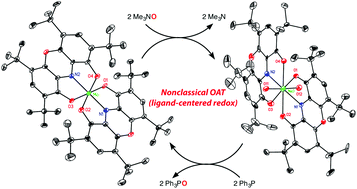
The dioxomolybdenum(VI) complex MoO2Cl2(dmf)2 reacts with Pb(DOPOQ)2 (DOPO = 2,4,6,8-tetra-tert-butyl-1,9-dioxophenoxazinate) to give MoO2(DOPOQ)2, which has an eight-coordinate structure with normal molybdenum-oxo bond distances and angles but elongated distances to the dioxophenoxazine ligand. The dioxo complex is deoxygenated by phosphines to produce octahedral Mo(DOPOCat)2, in which reduction has taken place at the ancillary ligands. This compound in turn reacts with trimethylamine-N-oxide to regenerate MoO2(DOPOQ)2, allowing a catalytic cycle for phosphine oxidation. This represents an example of four-electron nonclassical oxygen atom transfer in which both the oxidized and reduced forms of the metal complexes can be observed.


 Please wait while we load your content...
Please wait while we load your content...