Design, synthesis and comparison of water-soluble phthalocyanine/porphyrin analogues and their inhibition effects on Aβ42 fibrillization†
Abstract
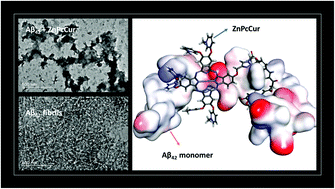
The misfolding and fibrillization of β amyloid (Aβ) is a major pathological hallmark of Alzheimer's disease (AD) and creates an important niche for developing targeted probe and drug designs. Phthalocyanine and porphyrin analogues are known to interact with Aβ species and interrupt their aggregation, and in this study we show that by conjugating with small molecules that can function as Aβ aggregation blockers such as curcumin and bexarotene, drug candidates with improved potential can be developed. In this work, we investigated porphyrin zinc (ZnPorp) analogues and phthalocyanine zinc (ZnPc) conjugates and compared their inhibitory effects on the formation of Aβ42 fibrils. We show that probe designs with a good hydrophilic–hydrophobic balance as observed with the ZnPc conjugate analogues are deemed as better inhibitors in modulating Aβ42 aggregation.


 Please wait while we load your content...
Please wait while we load your content...