Structural, spectroscopic, and computational evaluations of cation–cation and halogen bonding interactions in heterometallic uranyl hybrid materials†
Abstract
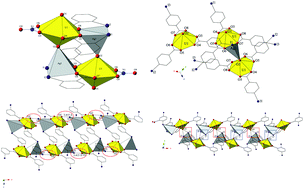
Harnessing the nominally terminal oxo atoms of the linear uranyl (UO22+) cation represents a frontier within the field of f-element hybrid materials. Here we outline a route for systematically accessing uranyl oxo atoms via judicious pairing with Ag+ cations or iodobenzoates, and describe the syntheses and crystal structures of four new heterometallic compounds containing Ag+ cations, the UO22+ cation, and o- (1), m- (2), p-iodo- (3), and 2,5-diiodo- (4) carboxylate ligands. Vibrational and luminescence spectroscopic properties for all four compounds are reported, as are computational findings from quantum chemical calculations and density-based quantum theory of atoms in molecules (QTAIM) analyses. Single crystal X-ray diffraction analysis of compounds 1–4 shows that the nominally terminal uranyl oxo atoms are engaged in either covalent UO2–Ag cation–cation interactions (1 and 3) or non-covalent assembly via halogen bonding interactions (2 and 4). Raman, infrared (IR), and luminescence spectra of 1–4 are redshifted with respect to the free uranyl cation indicating that both halogen–oxo and cation–cation interactions weaken the U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and in the case of 3 we note a rare example of activation of the uranyl asymmetric stretch (ν3) in the Raman spectra, likely due to the Ag–oxo cation–cation interaction lowering the symmetry of the uranyl cation. Quantum chemical calculations and QTAIM analysis highlight a quantitative difference between halogen bonds and cation–cation interactions, with the latter shown to significantly decrease uranyl bond orders and electron density at bond critical points.
O bond, and in the case of 3 we note a rare example of activation of the uranyl asymmetric stretch (ν3) in the Raman spectra, likely due to the Ag–oxo cation–cation interaction lowering the symmetry of the uranyl cation. Quantum chemical calculations and QTAIM analysis highlight a quantitative difference between halogen bonds and cation–cation interactions, with the latter shown to significantly decrease uranyl bond orders and electron density at bond critical points.


 Please wait while we load your content...
Please wait while we load your content...