The platination mechanism of RNase A by arsenoplatin: insight from the theoretical study†
Abstract
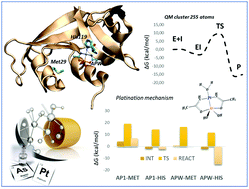
Herein, we present a detailed metalation process of the bovine pancreatic ribonuclease (RNase A) by a novel multitarget anti-cancer agent arsenoplatin-1, AP1, ([Pt(μ-NHC(CH3)O)2ClAs(OH)2]), on the basis of quantum chemical investigation, employing the B3LYP and M062x functionals and a large model of the active site. The proposed mechanism is consistent with the structural data. The role of water molecules in the active site is also analyzed. These studies revealed that the Nδ of His119 binds platinum(II), preserving the Pt–As bond. To better rationalize the different behavior of AP1 (bound to His119) and the reference cisplatin (bound to Met29) towards the same target RNAse A, also these processes have been considered. The final platinated complex structure agrees well with the crystallographic one. Our results evidence that the metalation process takes place more favorably in water than in the protein environment in agreement with the nature of the protein binding pocket residues.


 Please wait while we load your content...
Please wait while we load your content...