Oxidative esterification of aliphatic α,ω-diols, an alternative route to polyester precursors for the synthesis of polyurethanes†
Abstract
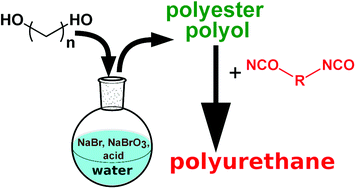
Polyester polyols synthesized via a polycondensation reaction of diols with diacids or their diesters are commonly used as polyurethane building blocks. Herein, we demonstrate an alternative route to polyester polyols via oxidative esterification of aliphatic α,ω-diols. Unlike the polycondensation reaction, this process is carried out in aqueous media at 30 °C under atmospheric pressure. In situ generated hypobromous acid from the mixture of NaBr, NaBrO3 and H2SO4 is used to trigger the reaction. The reaction progress can be conveniently controlled with the Br/OH molar ratio. The properties of the obtained polyesters are determined by the applied diol and can be tailored using a mixture of diols. The composition of polymers is determined with GPC, NMR, and MALDI-TOF MS. It is shown that polyesters obtained with this method can be used as precursors for polyurethane synthesis. This work provides a quick and convenient route to polyester polyol synthesis, feasible in any laboratory.


 Please wait while we load your content...
Please wait while we load your content...