Amphiphilic diblock copolymers of poly(glycidol) with biodegradable polyester/polycarbonate. organocatalytic one-pot ROP and self-assembling property†
Abstract
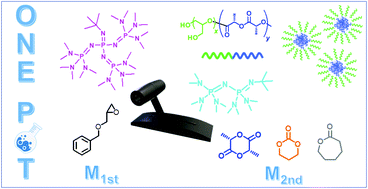
A synthetic method for a series of poly(glycidol) (PG)-based amphiphilic block copolymers is presented with an emphasis on the catalyst switch method from an organic superbase (t-Bu-P4) to another with reduced basicity (t-Bu-P2) during sequential ring-opening polymerization (ROP). This methodology allowed the first ROP of an epoxide monomer and the second ROP of cyclic ester monomers or a cyclic carbonate monomer to occur in a controlled manner, producing diblock copolymers with well-defined structures. Deprotection of the polyether segment resulted in amphiphilic block copolymers with PG as the effective hydrophilic segment and three different aliphatic polyesters or polycarbonate as the hydrophobic segments. The block copolymers’ physicochemical properties were systematically examined, demonstrating their excellent micelle-forming properties in aqueous media.


 Please wait while we load your content...
Please wait while we load your content...