Unprecedentedly high active organocatalysts for the copolymerization of carbonyl sulfide and propylene oxide: steric hindrance effect of tertiary amines†
Abstract
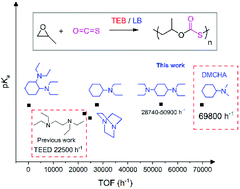
The copolymerization of carbonyl sulfide (COS) with epoxides has been developed to be a facile method to synthesize well-defined sulfur-containing polymers. This work describes the steric effect of tertiary amines on the copolymerization of COS with propylene oxide (PO) via a zwitterionic approach, using triethyl borane (TEB) accompanied by various tertiary amines. N,N-Dimethylcyclohexylamine accompanied by TEB (1/1) showed an exceedingly high turnover frequency of up to 69 800 h−1 and a copolymer selectivity of up to >99% for organocatalytic COS/PO copolymerization at 60 °C under solvent-free conditions. The obtained poly(propylene monothiocarbonate)s (PPMTC)s have fully alternating sequences, regioregularity of >99% tail-to-head (T–H) content, and high-number average molecular weights of up to 221.8 kg mol−1 and narrow dispersities (1.1–1.3) within 1 min. We disclosed that tertiary amines (pKa values range from 8.19 to 10.80) with less steric hindrance exhibited higher catalytic activity for COS/PO copolymerization.


 Please wait while we load your content...
Please wait while we load your content...