Supramolecular polymerization of BODIPY dyes extended with rationally designed pyrazole-based motifs†
Abstract
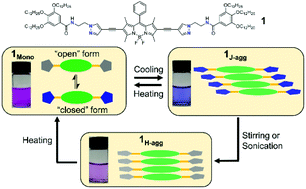
Controlled supramolecular polymerization has emerged as an active research area in the last decade. Recent contributions have revealed that their dynamic self-assembly into different molecular packings is strongly influenced by kinetic effects. However, the examples reported are very limited and the understanding of pathway complexity by molecular design remains elusive. In this work, two supramolecular monomers 1 and 2 by coupling BODIPY with a class of newly designed pyrazole-based motifs were designed. The flexible ethylene linker together with the presence of benzamide groups bring about intramolecular hydrogen-bonding (H-bonding) interactions between pyrazole N as the H-bonding acceptor and the adjacent amide. Our results unveiled two distinct pathways during self-assembly. Namely, a metastable J-aggregate is formed through an isodesmic model, while the thermodynamically stable H-aggregate obeys the cooperative mechanism. The detailed transformation of 1J-agg into 1H-agg, as well as the individual formation mechanisms of 1J-agg and 1H-agg have been investigated by various spectroscopic techniques. Encouraged by the drastic emission changes of monomers and aggregates, luminescence temperature sensing has been demonstrated as a proof of concept.


 Please wait while we load your content...
Please wait while we load your content...