Amino acid acrylamide mimics: creation of a consistent monomer library and characterization of their polymerization behaviour†
Abstract
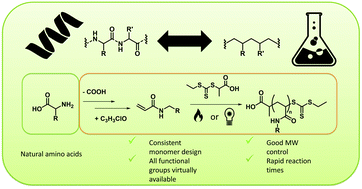
Many attempts to mimic the structure of biopolymers via precision polymer synthesis with reversible deactivation radical polymerization (RDRP) techniques have been made. For peptides and proteins and their building blocks in particular, a broad variety of mimics have previously been suggested. However, general consistency in the design of these materials has been lacking. In this work, the foundations of a consistent acrylamide monomer library that mimics essential amino acids has been laid, defining mimics as the reaction product of acryloyl chloride and the decarboxylized respective amino acid. Five of these monomers were then selected as model monomers, each representing one of the the five major groups of amino acids. The model monomers were synthesized in flow procedures directly from acrylic acid and subjected to reversible addition fragmentation chain transfer (RAFT) polymerizations. 2-Hydroxyethyl acrylamide, N-isobutylacrylamide, N-methylacrylamide and isopropyl 4 – acrylamidobutanoate (mimicking serine, valine, glycine and glutamic acid) were polymerized via a general thermal RAFT approach resulting in high monomer conversions and good molecular weight control after short reaction times of 5 to 15 minutes. For the mimic of arginine, 1,3-di-boc-guanidinobutyl acrylamide, photoRAFT at room temperature was required to reach consistent polymerization conditions, then also resulting in well-defined materials.
- This article is part of the themed collection: Molecularly Defined Polymers: Synthesis and Function


 Please wait while we load your content...
Please wait while we load your content...