Stereoselective ring-opening polymerization of functional β-lactones: influence of the exocyclic side-group†
Abstract
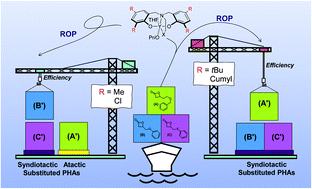
Polyhydroxyalkanoates (PHAs) are a unique class of polyesters especially due to their chemical diversity imparted by their side-chain substituent that provides a handle to tune their properties, such as their thermal, mechanical and (bio)degradability signature. Here, we report that some functional PHAs, namely PBPLFGs (FG = functional group), were synthesized by the controlled stereoselective ring-opening polymerization (ROP) of racemic 4-substituted-β-propiolactones, namely rac-BPLCH2ZPhs with Z = O, S, CH2OCH2, catalyzed by diamino- or amino-alkoxy-bis(phenolate) yttrium amido complexes (1a–1g) in the presence of isopropanol. Unprecedented syndio-enriched (Pr up to 0.87) PBPLCH2ZPhs of high molar mass (Mn,NMR up to 86 400 g mol−1, ĐM < 1.23) were thus typically prepared under mild operating conditions (toluene, 20 °C). Catalyst systems with smaller “uncrowded” Me or Cl ortho-substituents installed on the yttrium phenolate ligands (1a–1c) typically revealed less active than those with larger bulkier tBu or CMe2Ph groups (1d–1g), regardless of the monomer (TOF1a–1c = 0.48–15 h−1, TOF1d–1g = 450–1840 h−1). Irrespective of the catalyst system, exchanging oxygen with sulphur in BPLCH2ZPhs, with Z = O, S, did not affect the stereochemistry, always affording syndiotactic PBPLCH2ZPhs. On the other hand, either atactic or syndiotactic PBPLCH2CH2OCH2Phs were formed upon tuning the catalyst from 1a–1c or 1d–1g, respectively. The intimate relationship, through “non-covalent” interactions, between the chemical nature of the exocyclic functional side-group on the β-lactone and the stereoelectronic tuning arising from the phenolate ligand ortho-substituents within the yttrium coordination sphere, modulated the stereocontrol of the ROP. The thermal behavior of these original functional PHAs depended closely on their side-chain substituent (Tonsetd = 226 to 272 °C; Tg = −15 to +40 °C).
- This article is part of the themed collection: Sustainable Polymers


 Please wait while we load your content...
Please wait while we load your content...